Abstract
* A.M.Z. and M.S. contributed equally
Introduction: Response assessments in clinical trials and routine practice for patients (pts) with myelodysplastic syndromes (MDS) treated with hypomethylating agents (HMA) are based on the International Working Group (IWG) criteria proposed in 2000 and revised in 2006. However, it is not clear whether response criteria correlate with overall survival (OS).
Methods: We conducted a systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines with a systematic search using controlled vocabulary and free text terms for MDS and survival of Cochrane Library, Google Scholar, Ovid Embase, Ovid Medline, Pubmed, Scopus, and Web of Science Core Collection databases to find relevant articles published from inception to January 2022. The search was peer-reviewed by a second medical librarian. A targeted search of recent conference proceedings was also performed. Titles, abstracts, and full texts were reviewed for inclusion in the meta-analysis by two reviewers independently and conflicts were resolved by consensus discussion. Clinical trials and cohort studies with >100 pts reporting on the association of IWG 2000 or 2006 response criteria with OS among pts treated with HMA monotherapy or combinations were eligible for inclusion. Studies were excluded if they were (i) review or basic research articles, (ii) studies with <50% higher-risk MDS pts (inclusion of a minority of pts with MDS/MPN, oligoblastic AML, and lower-risk MDS was permitted), (iii) not published in English, and (iv) had insufficient reporting of the primary endpoint.
The primary endpoint was the hazard ratio (HR) for OS for patients achieving an IWG-defined response (complete remission [CR], marrow CR, partial remission, hematologic improvement [HI]) compared to patients not achieving a response. Random-effects models were used to pool the HRs for death. Cochran Q and I2 indices were used to assess heterogeneity across included studies. Study heterogeneity was graded based on I2 indices as low (I2 <30%), moderate (I2 =30-60%), or high (I2 >60%). Comprehensive Meta-Analysis (CMA version 2.2, Biostat) was used for all analyses.
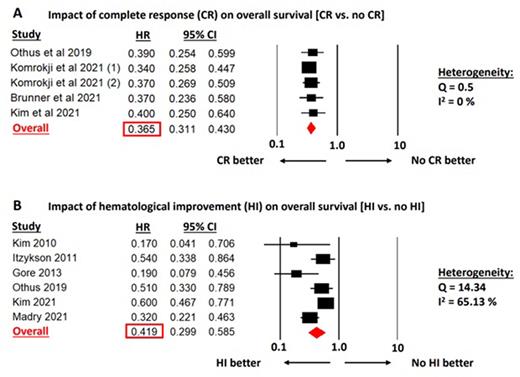
Results: Our search strategy identified 7023 studies after duplicate removal. Following abstract and title review to identify clinical trials and large cohort studies reporting IWG response and OS among MDS pts treated with HMA, 195 studies were assessed for eligibility as full texts. Among these 185 studies were excluded for the following reasons: insufficient reporting of the primary outcome of OS and IWG response (n=114), duplicate publications from the same pt cohort (n=42), <50% higher-risk MDS pts (n=13), cohort studies with <100 pts (n=6), not on HMA therapy (n=4), ongoing clinical trials without published results (n=3), transplant (n=2), and review article (n=1). The final set of studies included 3896 MDS pts treated with azacitidine, decitabine, guadecitabine, or HMA-combination in 9, 4, 1, and 2 studies, respectively.
Among the 5 available studies with data on the impact of CR on OS, the HR for death was 0.365 (95% CI: 0.311-0.430) for pts who achieved a CR compared to pts without a CR. Study heterogeneity was low (Cochran's Q=0.5; I2=0%; Figure A). Six studies reported the impact of HI on OS. Pts who achieved HI had favorable outcomes compared to pts without HI (HR: 0.419; 95% CI: 0.299-0.585) but with high heterogeneity across studies (Cochran's Q=14.34; I2=65.13%; Figure B). The prognostic impact of other response categories (e.g., marrow CR, partial remission) was only reported by 1 study each, precluding a meta-analysis.
Conclusion: Achieving CR as defined by the IWG MDS response criteria is associated with improved OS among higher-risk MDS pts treated with HMA-based regimens. HI was also associated with OS, but with high study heterogeneity. The strong and consistent association of CR with OS supports its use as an outcome in clinical trials in MDS. The prognostic implications of other response categories as such CR with partial hematologic recovery (CRh) especially with novel combination therapies require additional studies.
Disclosures
Platzbecker:BMS/Celgene: Honoraria; Jazz: Honoraria; Abbvie: Honoraria; Janssen: Honoraria; Silence Therapeutics: Honoraria; Takeda: Honoraria; Novartis: Honoraria; Geron: Honoraria. Fenaux:Novartis: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Celgene/BMS: Honoraria, Research Funding; Syros Pharmaceuticals: Honoraria. Sekeres:Bristol Myers-Squibb: Membership on an entity's Board of Directors or advisory committees; Takeda/Millenium: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Kurome: Membership on an entity's Board of Directors or advisory committees. Zeidan:Celgene/BMS, Novartis, Cardiff Oncology, AbbVie, Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Astex, Pfizer, Medimmune/AstraZeneca, ADC Therapeutics: Research Funding; Jazz, Agios, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, Beyondspring, Gilead, Kura, Tyme, Janssen, Syndax, Geron, Ionis, Epizyme: Consultancy, Honoraria; Novartis, Cardiff Oncology, Pfizer: Other: Travel Support; Gilead, Kura, Loxo Oncology: Consultancy, Honoraria, Other: Clinical Trial Committee; Celgene/BMS, AbbVie, Pfizer, Boeringer-Ingelheim, Trovagene, Cardiff Oncology, Incyte, Takeda, Novartis, Aprea, Amgen, Otsuka: Consultancy, Honoraria, Research Funding; Celgene/BMS, Novartis, AbbVie, Gilead, Kura, Loxo Oncology, Geron: Other: Clinical Trial Committee; Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Gilead, Kura, Loxo Oncology, Otsuka, Jazz, Agios, Acceleron, Astellas, Daiichi-Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Ionis, Epizyme, Janssen, Syndax, Genentec: Consultancy, Honoraria, Other: Advisory Boards; Astex, Medimmune, Astrazeneca, ADC Therapeutics: Research Funding; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie: Consultancy, Honoraria, Other: Advisory Board. Stahl:Novartis: Other: Advisory Board; Boston Consulting: Consultancy; Curis Oncology: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal